We Are So Close to Curing Cancer
The recipe includes international cooperation, an obsessed scientist and the New England Journal of Medicine.
After World War II, a civil war broke out in China leaving many Chinese citizens stranded in the United States including a young physician named Dr. Min Chiu Li ((李敏求). In the 1950’s, Li was recruited to the NCI to care for young women with a rare and uniformly fatal cancer called choriocarcinoma. Choriocarcinomas form in the uterus when cells that were part of the placenta begin to rapidly divide. Li watched these young mothers die in terrible pain as the cancer spread rapidly into their lungs and brain.
While Li was working at the NCI, reports began to surface about a chemotherapy agent called methotrexate which was being given to children with leukemia. Hoping for the same amazing results, Li began to give methotrexate to women with choriocarcinoma, closely monitoring their progress.
The first patient ever cured of choriocarcinoma was the 24-year-old wife of a US Navy dental technician who was near death after a tumor deposit of choriocarcinoma in her lung ruptured. Dr. Li went on to cure 2 other women before he was fired for continuing to give women chemotherapy after their symptoms resolved.
Li argued that their tumor markers were still elevated which indicated microscopic disease was still present, but to no avail. He eventually moved to Memorial Sloan Kettering Cancer Center where he continued his research and was vindicated when he received the Lasker Award (“America’s Nobel”) in 1972 for his work.
You can read more about Dr. Li in the post below which is the most viewed Cancer Culture post to date.
In the seventy-five years since Dr. Li’s discovery, chemotherapy cures have been discovered for non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, testicular cancer, some ovarian tumors and a few childhood cancers. These cancers, however, comprise a very, very small percentage of all cancers. They also use non-targeted chemotherapy agents mostly discovered in the 1980’s.
In 2001, Dr. Brian Druker ushered in the era of precision cancer medicine. Druker and colleagues published a report in the New England Journal of Medicine on their experience treating 83 leukemia patients with a new oral medication called imatinib or Gleevac. Gleevac targeted a mutation present in leukemic cells and produced amazing cures for an otherwise hopeless disease. The drug’s success appropriately landed it on the cover of Time magazine.
Fear The Walking Dead
This week, the deliciously honest actor, writer and artist Mo Collins shares her experience of being diagnosed with and treated for a GI Stromal Tumor (commonly called GIST).
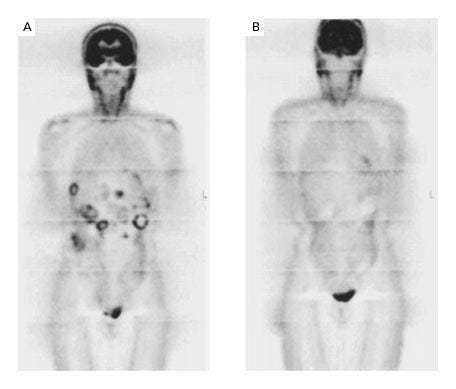
Researchers in the US and Finland soon found that cells in a rare type of sarcoma, called a Gastrointestinal Stromal Tumor or GIST, were also sensitive to Gleevac. The first patient with GIST to receive Gleevac was a 50-year-old Finnish woman whose cancer had progressed through surgery, chemotherapy, and other treatments.
After one month on the drug, her PET scan was clear (below). Eight months later, most of her liver metastases had completely disappeared. For the first time, a treatment targeting a specific mutation cured a solid tumor. In 2002, a case report of her treatment was published in the New England Journal of Medicine. Phase II trials tested the dosing of the drug which is now standard of care.
Historically, the survival of a patient with advanced GIST was less than a year. Patients now live for decades by taking a pill a day. A recent review article characterized the use of Gleevac to treat GIST as “the most successful application of targeted therapy for treatment of a solid cancer.”
I agreed…until last month.
At the American Academy of Cancer Research annual meeting, researchers from Memorial Sloan Kettering Cancer Center (MSKCC) brought us even closer to a cure. Not just for rare cancers like GIST and choriocarcinoma but for one of the most common cancers diagnosed in the world AND one whose incidence is rising in young people.
How young is 'too young' for cancer screening?
As many of you may know cases of colorectal cancer in adults under 50 are increasing. Individuals born in 1990 have double the risk of colon cancer and 4x the risk of rectal cancer than people born in 1950.
Three years earlier, this team from MSKCC, led by Dr. Luis Diaz, had published a report in the New England Journal of Medicine. The findings were so remarkable that my college-age stepdaughter forwarded me a TikTok video that came across her feed. She wondered if I’d heard about this study.
In their initial study, twelve patients with advanced rectal cancer were treated with a new drug called dostarlimab which targeted a mutation found in their tumors. The patients on this trial experienced an unprecedented 100% tumor control with the experimental medication alone. No surgery. No radiation. No chemotherapy.
Every single patient on the trial avoided the life changing consequences of typical treatment including colostomies, impaired bowel function and infertility. One patient who was diagnosed and treated shortly after the birth of her first child went on to give birth to 2 more, an unthinkable possibility with standard treatment.
Needless to say, the AACR presentation hall was full as the Diaz team stepped onto the stage to present their most recent results. This time they had studied dostarlimab in patients with other advanced cancers including esophageal and prostate cancer. They had also followed patients for a longer period of time.
The results, published concurrently in the New England Journal of Medicine, were shockingly similar to the results from three years prior. Every patient with rectal cancer had a complete response as did most colon and gastric cancer patients.
Over 80% of patients were cured with the medication alone. Most patients escaped the life changing consequences of standard treatment.
Even more amazing was that over a third of patients on the study had NO side effects. Zero.
I can’t overstate how brave what the MSKCC team and patients did in this trial was. It is one thing to sign up patients with an aggressive cancer to try adding another treatment on to standard of care. But to look a patient in the eye and ask them to trust you with a new treatment when there’s an alternative that’s stood the test of time? That, excuse my language, takes balls.
In an interview, Diaz acknowledged the bravery of the patients on the study, the first to attempt to cure solid tumors without surgery, “This is how cancer’s been treated for hundreds of years, and we’re going to say, ‘You know what, we’re going to take a shot and not do that,’” Diaz said. “Not doing surgery is very gutsy, super gutsy.”
As Diaz explained, “Think of the evolution of the radical mastectomy, to the lumpectomy, to the optional lymph node dissection, to not doing lymph node dissection. Oncologic care has always gotten smaller. I think we’re seeing the same sort of trend; where we can minimize the brutality of some of the things we do and lead to better outcomes.”
Despite his contributions and clear understanding of the future of cancer research, Diaz was removed from the National Cancer Advisory Board last week. Appointed by President Biden to serve a 7-year term in 2021, Diaz was part of this small group of cancer experts who advise and assist the NCI director in shaping the direction of the national cancer research program. Diaz was the only member to be removed.
Those of you who listened to my podcast will recognize this story, the pursuit of less radical, more personalized treatment…and the political consequences for doing so.
Episode 1: Who is Bernie Fisher?
On a fall weekend in late September 1974, a former dancer from Michigan and a young surgeon from Pittsburgh met just outside Washington, DC. The treatment of breast cancer would never be the same.
You are going to hear alot in the upcoming months about the necessity of performing randomized, control trials. That these are the “gold standard” by which every new treatment must pass. These pronouncements should be viewed with skepticism. For if we evaluate the efficacy of new treatments only by comparing them to standard therapies, our progress will be slow. Too slow for most of us to reap the rewards.
The end of cancer will not be ushered in by results from a Phase III randomized trial. It will also not be funded by a large pharma company or spearheaded by a scientist with a large social media presence. It will, however, follow the pattern illustrated above.
A small Phase I or II study will be reported in the New England Journal of Medicine. There will be no control arm because to do so would be unethical. Evaluating the options presented to them, patients will refuse to be randomized to a surgery arm. Based on Diaz’s results, I can’t say I would blame them.
The trial will be international collaboration of scientists, physicians, manufacturers and/or patients. China, Finland, New York, Washington, DC, California, Michigan, Ohio and so many more will contribute.
The effort will be spearheaded by an obsessed scientist with disruptive ideas. Diaz once told an interviewer, “I remember feeling like a weirdo back in college. I [would] spend nights and weekends in the labs. Even when friends would celebrate after an exam, I would go right back to the laboratory.” MC Li was fired for his brilliance. We must support the mavericks who will save us.
Physician-scientists began writing the playbook for ending cancer after World War II. If we are to see their work to its conclusion, we must listen to what they had to say and follow the path they set before us. It will lead us to the end of cancer if we only have the courage to continue.










Thank you, Dr. Wentworth, for this deeply inspiring and historically grounded piece.
As someone who has spent the last decade developing an alternative, targeted, and non-systemic cancer therapy, I see your article as both a validation and a call to action.
Your recounting of Dr. Min Chiu Li’s story and the boldness of Dr. Diaz’s recent work resonates deeply with me. Like them, I have faced skepticism, regulatory inertia, and isolation — yet continued because I see patients, real patients, responding to something the system isn't yet ready to embrace.
I invented **Intra-Tumoral Chlorine Dioxide Injection Therapy**, a minimally invasive approach that uses localized oxidative pressure to selectively destroy solid tumors without harming healthy tissue. We do not rely on systemic toxicity. We use image-guided precision to inject directly into the tumor — and we see results: breast cancer, liver cancer, peritoneal metastases, all responding rapidly.
We have now treated patients in Germany, China, and Spain with consistent outcomes, and are preparing data for publication. Like Dr. Diaz’s trial, many of our cases cannot ethically be randomized: patients refuse surgery and chemo, and there is simply no comparable control to “not injecting the tumor.”
What I see is a clear pattern: progress does not follow the establishment. It follows logic, courage, and patients who are willing to try when institutions are not.
Thank you again for articulating this vision. We are much closer than people think — and as you said, only need the courage to continue.
— **Xuewu Liu**
Inventor, Intra-Tumoral ClO₂ Therapy
cdsxcancer.com | clo2xuewuliu.substack.com
This is incredible. I cannot believe this was possible. This should be everywhere on the news. I am sharing this with people. Drugs that effective should never be ignored.